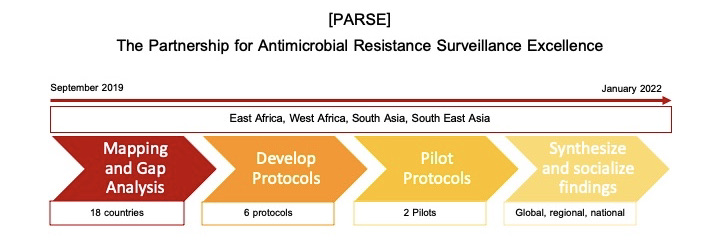
CORDS is the recipient of a Round 2 Fleming Fund Regional Grant. The aim of this grant is to strengthen AMR surveillance through the development and piloting of common surveillance protocols through the Partnership on AMR Surveillance Excellent (PARSE). Prior to protocol development and testing, it is key to conduct a situational analysis and identify gaps in existing antimicrobial surveillance systems in the four Fleming Fund priority regions: East Africa, West Africa, South Asia, and Southeast Asia. Following this mapping of existing gaps and challenges, partners will work together to develop evidence-based AMR surveillance protocols, pilot them across the regions, and share results with key stakeholders.
Technical Steering Committee
The Technical Steering Committees (TSC) provides oversight of the implementation of two AMR surveillance protocols, one in Ghana and one in Tanzania over the period of April 2021 to January 2022. The TSCs make recommendations on technical aspects of protocol implementation and identify needed course corrections for quality and timely completion. The TSCs contribute to an expansion of partnerships and collaborative work environments and provide insight about issues faced by collaborating institutions and partners.
TSC members for protocol piloting in Ghana
Protocol: One Health surveillance of AMR in non-typhoidal Salmonella, Klebsiella pneumoniae, and other gram negative bacteria
- Nigel French, Animal Health Specialist and Fleming Fund Expert Advisory Group (FF EAG) member (New Zealand)
- Joanna McKenzie, One Health Specialist (New Zealand)
- Daniel Cohen, Lead on Pilot (Tel Aviv, Israel)
- Claire Gordon, Lead Clinical Microbiologist (London, UK)
- Perdita Hilary Lopes, Regional One Health Coordinator (Accra, Ghana)
- Ghana Country Grantee tbc (Accra, Ghana)
- Natalie Vestin, Antimicrobial Stewardship and Communications Specialist (Minneapolis, USA)
- Claudia Parry, Health Specialist and RGC (London, UK)
- Mark Smolinski, Medical Epidemiologist (San Francisco, USA)
- Louise Gresham, Epidemiologist (Washington DC, USA)
TSC for protocol piloting in Tanzania
Protocol: Surveillance of multi drug resistant bacteria causing community acquired urinary tract infections
- Antoine Andremont, Professor of Microbiology and FF EAG member (Paris, France)
- Mark Rweyemamu, SACIDS/CORDS, One Health Specialist (Dar es Salaam, Tanzania)
- Mecky Matee, Lead on Pilot (Dar es Salaam, Tanzania)
- Emmanuel Azore, Regional Microbiology/Laboratory Specialist (Kampala, Uganda)
- Claire Gordon, Lead Clinical Microbiologist (London, UK)
- Claudia Parry, Health Specialist and RGC (London, UK)
- Mark Smolinski, Medical Epidemiologist (San Francisco, USA)
- Louise Gresham, Epidemiologist (Washington DC, USA)
Protocol Development and Piloting
Based on mapping and gap analysis results and review of existing guidelines PARSE partners developed common protocols and SOPS to address AMR surveillance gaps. These protocols center the One Health approach and cross sectoral engagement.
Two protocols were selected for piloting:
- A protocol to monitor community-acquired UTI for AMR is being piloted in Dar es Salaam and Mwanza, Tanzania by the SACIDS Foundation for One Health, led by partners at Catholic University for Health and Allied Sciences, Muhimbili University for Health and Allied Sciences, and the Ministry of Health.
- A protocol to monitor resistance in non-typhi salmonella and klebsiella in humans and poultry is being piloted Kumasi and Accra, Ghana by the Middle East Consortium for Infectious Diseases, led by partners at Tel Aviv University and the University of Ghana.
Key AMR stakeholders are engaged at the regional and national levels to ensure buy-in, acceptability, and sustainability.
Results Sharing
Once PARSE pilots of common protocols are complete, they will share results with national, regional, and global stakeholders in the AMR sphere. Based on findings and discussions, PARSE Partners will make recommendations about the adoption and sustainability of the piloted AMR surveillance protocols.
Recent Activities
On July 1, 2021, the Partnership on AMR Surveillance Excellence (PARSE) team in Tanzania held a national meeting to socialize the protocol intended for piloting, on the Surveillance of multi drug resistant bacteria causing community acquired urinary tract infections. The Tanzania PARSE team is comprised of AMR experts from the SACIDS Foundation on One Health, Catholic University of Health and Allied Sciences, Muhimbili University of Health and Allied Sciences, Sokoine University of Agriculture, and the Tanzania National Public Health Laboratory. Attendees included representatives of these organizations as well as PATH/IDDS, the Ministry of Health, Kilimanjaro Christian University Medical College, the National AMR Surveillance and Research Technical Working Group, and the AMR Multisectoral Coordinating Committee. The protocol pilot began in Dar es Salaam and Mwanza, Tanzania in early July and results are expected at the end of 2021.

Image: Group photo PARSE national meeting, Tanzania, 1 July 2021.







